About The Group
Nordic Pharma is an international marketing and sales company with a history of internal product development and acquisitions.
Through a combination of innovation, collaboration and quality, Nordic Pharma strives to address specific unmet medical needs.
Nordic Pharma holds an established position in Women’s Health, a fast-growing Rheumatology franchise with a global footprint, and an increasing international presence in Ophthalmology.We bring over 25 years of expertise since our creation in 1995.


Our organization
Today, Nordic Pharma is:
- 300+
Employees
worldwide - 20
Different
countries - 03
Continents – and
counting
We are a medium-size pharmaceutical company with an entrepreneurial spirit. As such, we promote change and encourage the development of new ideas. We create opportunities for personal and professional development.
Portfolio of therapeutic areas
Nordic Pharma aims at developing products that address specific unmet medical needs of healthcare professionals and patients. Today, we have a range of highly specialized proprietary and in-licensed products in the following therapeutic areas: Women’s Health, Rheumatology, Ophthalmology, Critical Care, Others.
WOMEN'S HEALTH
RHEUMATOLOGY
OPHTHALMOLOGY
CRITICAL CARE
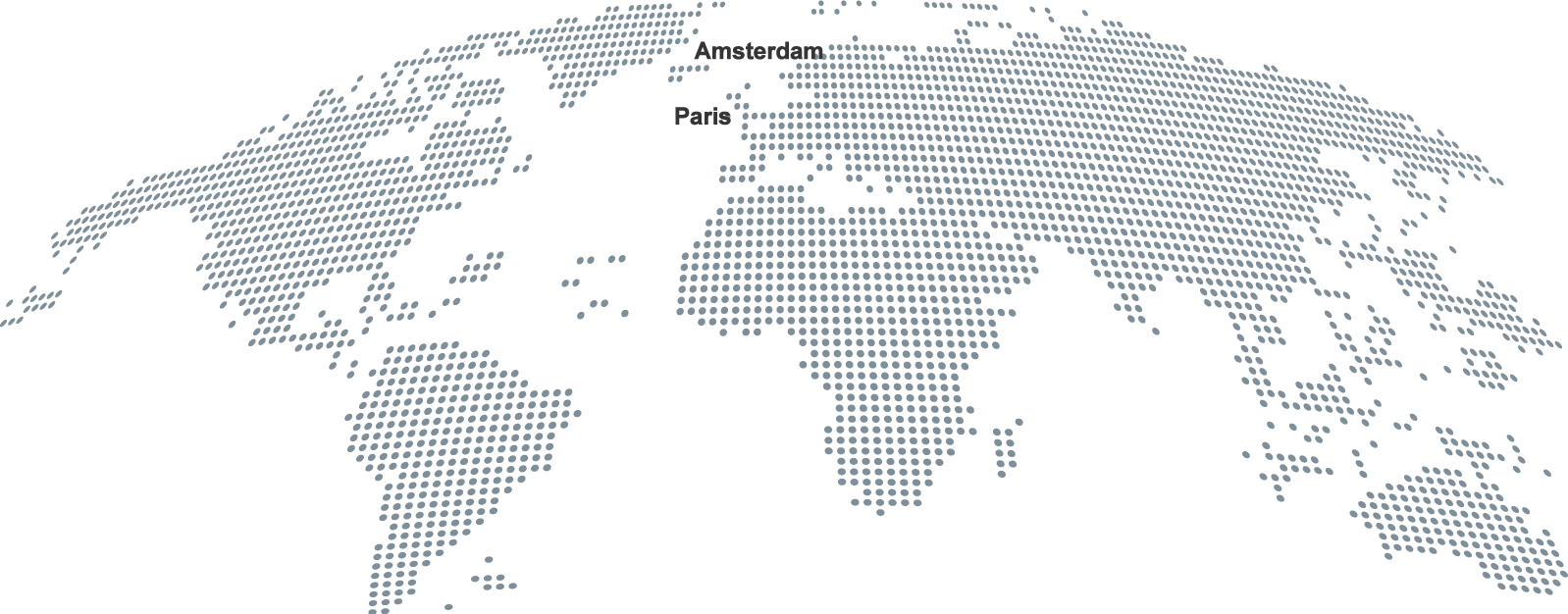
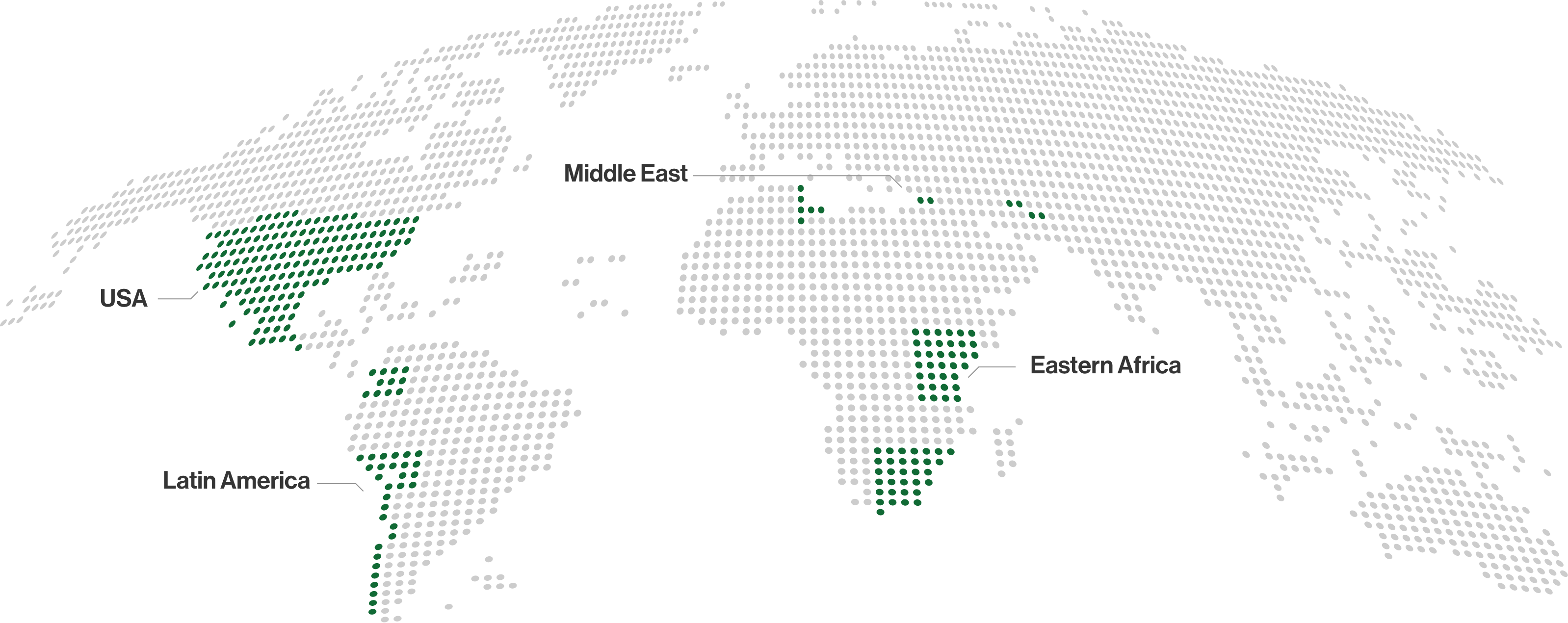
Our Presence
Nordic Pharma has affiliates in most European countries and has established a global footprint with key strategic partnerships to promote and distribute our products worldwide.
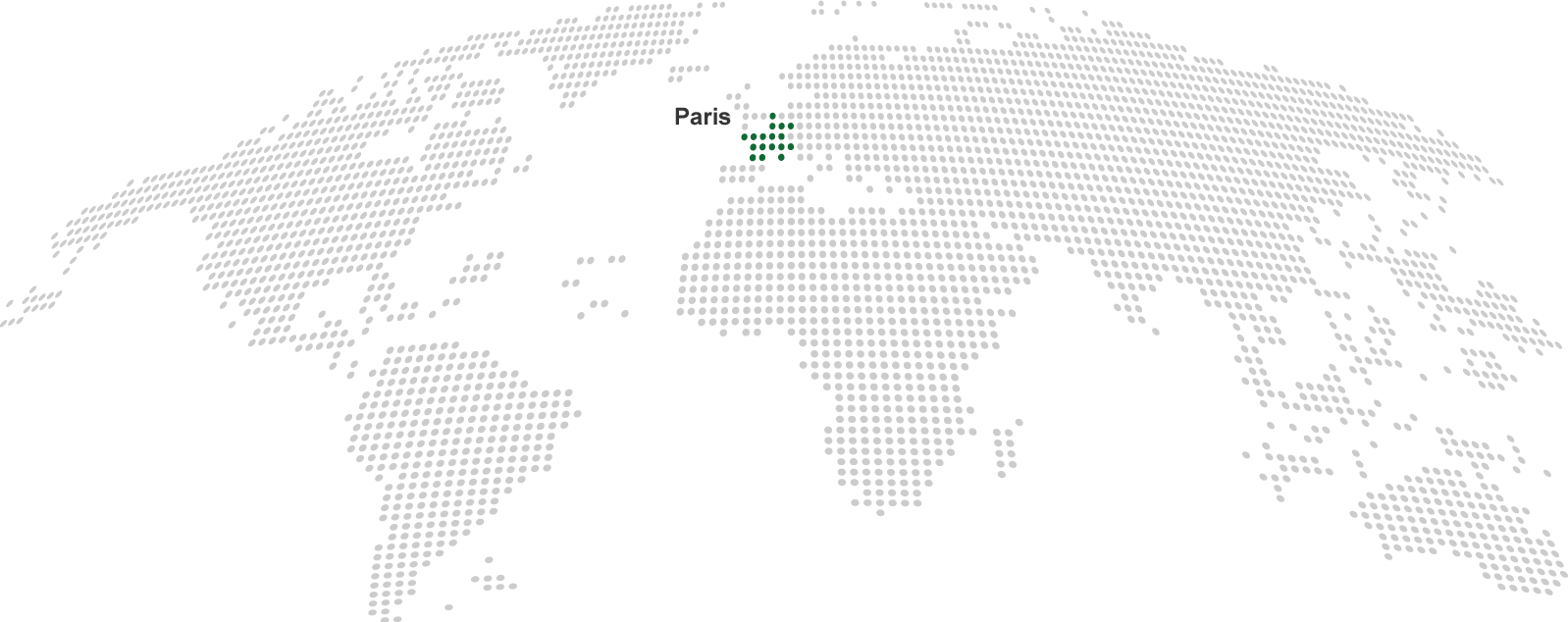
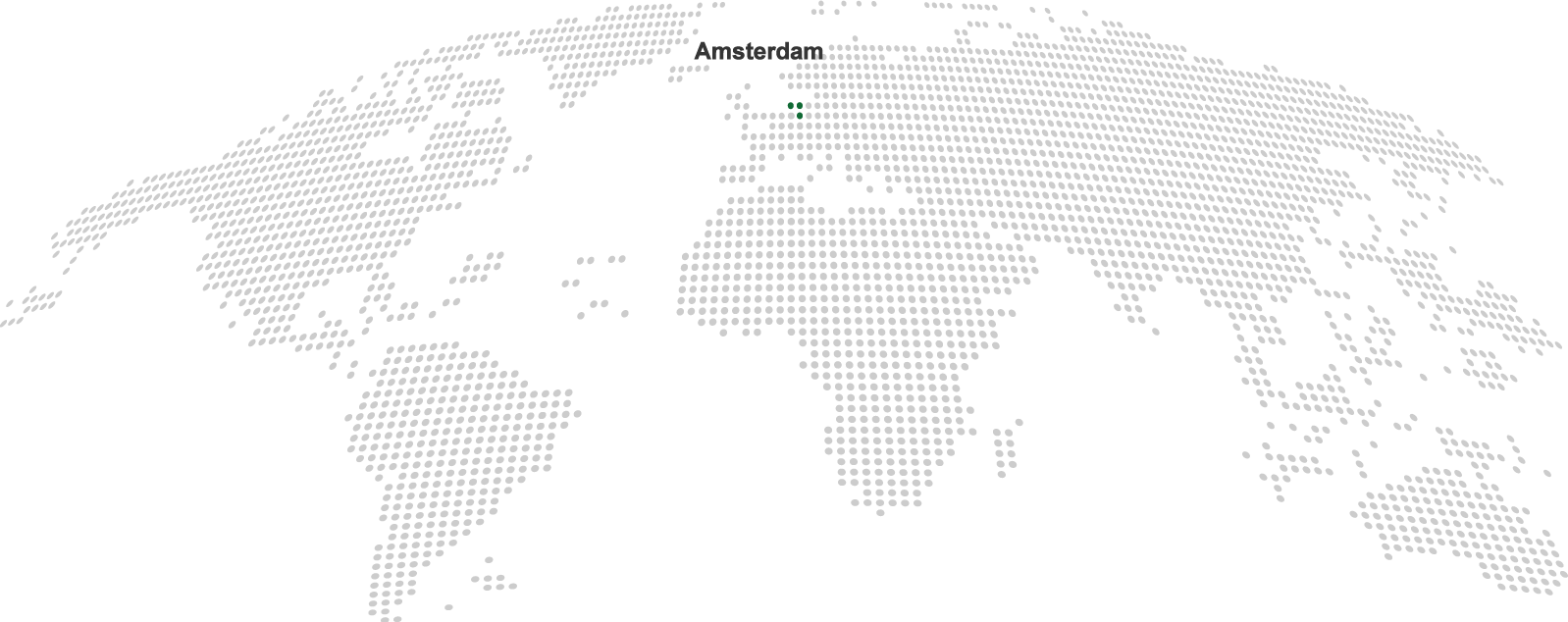
| Our headquarters (2) |
| Our affiliates (12) |
| Our commercial partners |
Nordic Pharma
Nordic Group BV
216 Boulevard Saint Germain
75007 Paris
France
Tel: +33 (0)1 70 37 28 02
Nordic Pharma
Nordic Group BV
Siriusdreef 41
2132 WT Hoofddorp
The Netherlands
Tel: +31 (0)85 48 35 871
Nordic Pharma Netherlands
Nordic Pharma B.V.
Siriusdreef 41
2132 WT Hoofddorp
The Netherlands
Tel: +31 (0)85 48 35 871
www.nordicpharma.nl
Nordic Pharma France
Nordic Pharma SAS
251 Boulevard Pereire
75017 Paris
France
Tel: +33 (0) 1 70 37 28 00
www.nordicpharma.fr
Nordic Pharma Americas, Asia, Pacific
Nordic Pharma Inc.
www.nordicpharma.ca
Nordic Pharma Central Eastern Europe
7, Mircea Eliade Blvd
Bucharest
Romania
Tel: +40 31 620 1204
Nordic Pharma Italy
Nordic Pharma S.r.l
Strada Anulare Torre 10
20090 San Felice – Segrate (MI)
Italy
Tel: +39 02 7532629
www.nordicpharma.it
Nordic Pharma Spain
Nordic Pharma SAU
Adolfo Pérez Esquivel, 3. 2°.
Of. 17. Edificio Las Américas
III. 28232 Parque Empresarial
Las Rozas, Madrid, Spain
Tel: +34 916 404 041
www.nordicpharma.es
Nordic Pharma Switzerland
Nordic Pharma GmBH
Binzmühlestrasse 80, 8050
Zürich
Switzerland
Tel: +41 43 444 92 91
www.nordicpharma.com/nordicpharma-ch
Nordic Pharma Ireland
Nordic Pharma Ltd
Allphar Services Ltd, 4045
Kingswood Road, Citywest
Business Park, Co. Dublin
Ireland
Nordic Pharma UK
Nordic Pharma Ltd
Building 1410 Arlington Business Park
Theale,
Reading, RG7 4SA
United Kingdom
Tel: +44 (0)118 221 0150
www.nordicpharma.co.uk
Nordic Pharma Germany + Austria
Nordic Pharma GmBH
Fraunhoferstraße 4 D-85737
Ismaning
Germany
Tel: +49 (0) 89 88 96 90 68 -0
www.nordicpharma.de
Nordic Pharma Scandinavia
Nordic Drugs AB
Box 300 35, 200 61
Limhamn
Sweden
Tel: +46 40 36 66 00
www.nordicdrugs.se
Nordic Pharma Belgium + Luxembourg
Nordic Pharma N.V.
Garden Square, Laarstraat 16
2610 Wilrijk
Belgium
Tel: +32 (0)3 820 52 24
https://www.nordicpharma.be/